For each question, enter the correct numerical value (in decimal notation, truncated/rounded-off to the second decimal place; e.g. 6.25, 7.00, -0.33, -.30, 30.27, -127.30) using the mouse and the on-screen virtual numeric keypad in the place designated to enter the answer.
The plot given below shows 𝑃 − 𝑇 curves (where P is the pressure and T is the temperature) for two solvents X and Y and isomolal solutions of NaCl in these solvents. NaCl completely dissociates in both the solvents.
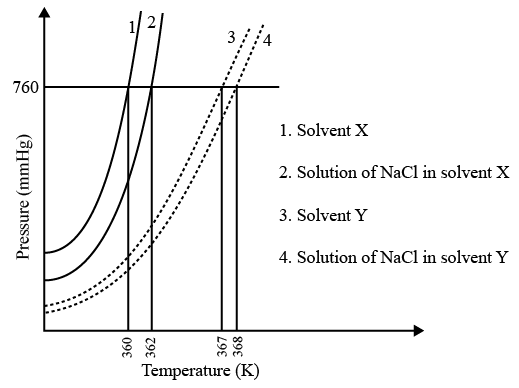
On addition of equal number of moles of a non-volatile solute S in equal amount (in kg) of
these solvents, the elevation of boiling point of solvent X is three times that of solvent Y.
Solute S is known to undergo dimerization in these solvents. If the degree of dimerization is
0.7 in solvent Y, the degree of dimerization in solvent X is ____.
Correct Answer: e
Create a FREE account and get:
- Free JEE Advanced Previous Papers PDF
- Take JEE Advanced paper tests
